20/04/2023
Ottawa, Ontario — Thursday April 20, 2023

The CHEO Research Institute (RI) has received funding from the New Frontiers in Research Fund (NFRF) – 2022 Horizon Global Platform Program to participate in the recently announced 8.8 million euro SIMPATHIC consortium established under the European Union’s ‘Horizon Europe’ funding stream to accelerate therapeutic development for patients in Canada and around the world with neuromuscular diseases.
The Lochmüller Lab and CHEO Research Institute role in the SIMPATHIC consortium
Through the Lochmüller Lab and Dr. Alex MacKenzie‘s research group, the CHEO RI brings to the consortium unique and globally leading expertise in congenital myasthenic syndromes (CMS) and myotonic dystrophy, including experience with patient cohorts from across Canada. The RI’s role in the project will be to establish cell models from patients with CMS and myotonic dystrophy and to apply robust molecular and cellular measurements that reflect neurological symptoms. These measurements will validate ‘top hits’ from drug screens to identify promising drug candidates for clinical trials.
Following the initial stage of the project, the CHEO RI will participate in the design and execution of a novel basket trial, which will include patients who have different genetic diagnoses but overlapping clinical and molecular characteristics. This will allow the consortium to repurpose drugs simultaneously to more than one rare disease, an approach that has been met with some success in cancer. The CHEO RI will draw on the patient population here in Canada to recruit a cohort of patients to take part in this trial.
“Particularly in rare diseases, research has to transcend national barriers, as patients are scarce and collaboration across the world is essential to progress towards therapies. We are thrilled to have this opportunity to be part of the SIMPATHIC consortium. It will allow young scientists in our lab to work with Europe’s leading researchers, developing an understanding of how collaborative research works as well as gaining an international career profile, and it gives us the opportunity to share our lab’s expertise in neuromuscular disorders with this important effort to repurpose existing drugs for these incurable conditions,” said Hanns Lochmüller, Senior Scientist at the CHEO RI and Professor of Neurology, University of Ottawa Faculty of Medicine.
A unique opportunity for transnational collaborative research including Canadian researchers and patients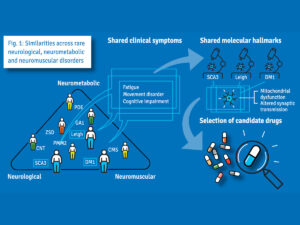
The 100 billion Euro Horizon Europe programme, run by the European Commission until 2027, is the largest ever transnational programme supporting research and innovation. Participating in European projects is not always easy for Canadian researchers, as international funding streams like Horizon Europe are not usually open to Canadian participants. The co-funding awarded by NFRF enables Canadian groups like the Lochmüller Lab to take part in this major consortium as equal partners.
About the SIMPATHIC Consortium
The SIMPATHIC Consortium, led by the Dutch Radboud University Medical Center and Amsterdam UMC, has developed a new approach to expedite the use of existing drugs for groups of patients with rare neurological disorders. The consortium has been awarded an 8.8 million euros ($12.9 million CAD) grant from the Horizon Europe program to further develop this innovative method.
Traditionally, drugs are developed one disease at a time, which is costly and time-consuming. It often takes a long time before patients can use a new drug. The international SIMPATHIC Consortium, which comprises 22 international partners, has created a novel method for accelerating the use of existing drugs for other conditions, based on screening tissues from individual patients. The consortium’s approach has been recognized and funded by the European Commission with a 8.8 million euros grant.
The researchers will use a new technology to test the efficacy of existing drugs in patients with neurological disorders, requiring only a tube of blood or a small piece of skin from the patient. These materials contain stem cells that the researchers culture into nerve cells. They subsequently test how they respond to a variety of existing drugs.
If the researchers observe a positive effect of a drug, they will launch a clinical study in a group of patients with different disorders but similar clinical symptoms. As existing drugs have already been tested in humans, animal studies may not be necessary, which significantly accelerates the use of drugs in new applications and reduces research costs.
Project leader and bioinformatician Prof. Peter-Bram ‘t Hoen of Radboudumc explains, “The project will significantly improve the efficiency and speed of discovery, evaluation, and approval of new applications for existing drugs.”
Project leader and pediatrician-biochemical geneticist Prof. Clara van Karnebeek of Amsterdam UMC adds: “In addition, it will have a huge impact on the care of patients with rare neurological disorders, for example, by accelerating drug availability for these debilitating conditions.”
The original press release from Amsterdam University Medical Centre is available here.
-30-
Media contact:
Jennifer Ruff
Director of Communications
CHEO Research Institute
(613) 261-3979
[email protected]
